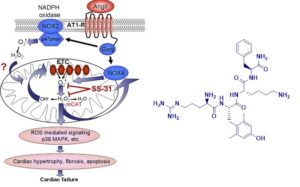
The recent development of a series of mitochondrial targeted peptides that can inhibit MPT provides an opportunity to examine the potential of MPT inhibition as an approach to the treatment of PD. The SS (Szeto-Schiller) peptides are cell-permeable synthetic tetrapeptides that selectively partition to the inner mitochondrial membrane. Although these peptides carry 3+ net charge at physiologic pH, their mitochondrial uptake is not dependent on mitochondrial potential, and they do not cause mitochondrial depolarization. These mitochondrial-targeted peptides decrease mitochondrial ROS production, and inhibit mitochondrial swelling and cytochrome c release in isolated mitochondria. The inclusion of a tyrosine or modified tyrosine residue provides additional free radical scavenging properties, and these analogs are very potent in ameliorating ROS-induced cell death and ischemia-reperfusion injury. Surprisingly, even the analogs that do not possess intrinsic scavenging ability can significantly reduce oxidative stress and prevent ischemia-reperfusion injury. The Szeto-Schiller (SS)-31 peptide (D-Arg-2′,6′-dimethyl-tyrosine-Lys-Phe-NH2) belongs to a family of aromatic cationic peptides that selectively target to mitochondrial inner membrane and can scavenge superoxide, hydrogen peroxide, peroxynitrite, and hydroxyl radicals. The SS-31 has been shown to reduce mitochondrial ROS in epithelial, endothelial, and neuronal cells exposed to electron-transport-chain inhibitors as well as pro-oxidants including t-butyl-hydroperoxide and hypochlorous acid. The SS-31 also inhibits apoptosis mediated by mitochondrial release of cytochrome c elicited by mitochondrial permeability transition. In vivo administration of SS-31 has demonstrated efficacy in several animal models associated with mitochondrial oxidative stress, including reduction of ischemia-reperfusion injury, protection against neurodegeneration, and prevention of insulin resistance induced by high-fat diet.