$ 720.0
Quantity: 1Gram
In stock
Description
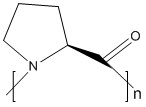
Product name: Poly-L-proline
Catalog#: 1505606
Cas#: 25191-13-3
M.W.: >10K
Format: Hygroscopic, yellow to brown solid
Description: Poly-L-Proline is known to form helical structures with two well-characterized conformations: a left-handed Poly-L-Proline helix (PPII) is formed when the sequential residues all adopt backbone dihedral angles (ϕ,ψ) of (−75°,146°), with all prolyl bonds in the trans-isomer conformation (i.e. backbone dihedral angle ω = 180°) with 3 residues per turn; and a more compact right-handed Poly-L-Proline helix (PPI) is formed with all sequential residues adopting dihedral angles of roughly (−75°, 160°) and all prolyl bonds assume a cis-isomer conformation (i.e. backbone dihedral angle ω = 0°) with 3.3 residues per turn.The lone natural N-substituted polypeptide is poly(l-proline) (PLP), which is the only pH-independent, aliphatic, water-soluble, helical polypeptide. It is characterized by hydrophilic interactions (hydrogen bond donation by water molecules to both the tertiary amine and the carbonyl group) and hydrophobic interactions (pyrrolidine rings). Due to PLP’s rigid backbone, containing consecutive pyrrolidine rings and resonance-stabilized imide peptide linkages, it can serve as a scaffold for protein–protein recognition, molecular identification, enzyme tetramerization, and cell penetration, while the large effect of the polyproline helix on the protein conformation renders PLP a useful model for the study of native proteins such as gelatin and collagen.
Usage: For Scientific Research Use Only, Not for Human Use.