Therapeutic peptides are described as naturally occurring short amino acid monomer chains, shorter than 100 amino acids, and they act by binding to specific cell surface receptors, where they trigger intracellular pathways. They have been shown to possess desirable pharmacological profiles and their specificity has been seen to translate into outstanding safety, tolerability, and efficacy profiles in humans, in stark contrast to traditional small molecules. The idea of using peptides as therapeutic agents has been historically ignored by pharmaceutical companies due to several limitations including size, which makes them very susceptible to degradation by peptidases, the lack of effective methods for delivery, poor transport properties through biologic membranes, low oral bioavailability, rapid excretion, and poor target specificity resulting from the flexible nature of peptides. More recently, however, in light of advances in processing technologies, there has been a renewed interest in peptides and peptidomimetics as potential therapeutic agents. This is partly due to numerous improvements made to stability, transport, affinity profiles, and oral availability. Furthermore, the introduction of alternative delivery methods by new adjuvant and carrier systems have been developed, and the advance of proteomics identifying innumerable PPI targets, has increased the interest in peptides and their mimetics as potential therapeutic drugs.
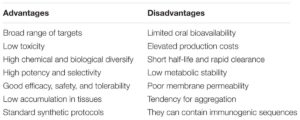
To address these key technical hurdles to use peptides as medicines, a number of modifications strategies (thanks to robust peptide-chemistry approaches developed in recent years) have been widely adopted. Several bioactive peptides have proven to be highly functional with many serving as potent agonists and antagonists against numerous receptors implicated in disease progression. Transformation of peptides to peptidomimetics is one intriguing mechanism to use peptide sequences as potential therapeutic agents. Peptides can be adapted to stable mimics that expose similar effects to their peptide analog but show increased consistency in structure, more target specificity, increased stability to proteolytic digestion and greater cell membrane permeability. Therefore, peptides have been chemically altered to include unnatural amino acid substitutions, backbone amide bond modifications, or rigid scaffolds or the addition of hydrophobic residues. Recently, development of orally and nasal active preparations have been proposed as a result of encapsulation of peptides in nanoparticles (e.g., liposomes, synthetic polymers, or fullerenes) which shields the drug from protease digestion until required, therefore increasing stability. Other strategies under investigation to overcome peptide barriers include use of protease inhibitors, absorption enhancers, or conjugated molecules in combination with the peptide structure, such as antibodies to improve targeting, carbohydrates to increase solubility, or lipids to enhance peptide permeability.