$ 300.0
Quantity: 1Gram
In stock
Description
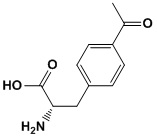
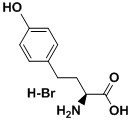
Product name: p-AcPhe(Acp)
Synonym: (S)-3-(4-acetylphenyl)-2-aminopropanoic acid,4-Acetylphenylalanine,p-Acetylphenylalanine,4AF, L-4-acetylphe,
Catalog #: 8215112
Purity: 95%
CAS No.: 122555-04-8
Molecular Formula: C11H13NO3
Molecular Weight: 207.23
Compound ID: 6420123
Description: The incorporated UAA p-acetylphenylalanine (p-AcPhe) acts as a reactive chemical handle enabling site-specific direct covalent coupling to the Fab. The ketone reacts with hydrazines or aminooxy groups to produce a hydrazone or oxime adduct, respectively. Site-directed spin labeling of the unnatural amino acid p-acetylphenylalanine (p-AcPhe) using oxime based coupling chemistry is successfully applied to investigate human sulfite oxidase (hSO), a protein containing an essential cysteine residue, which impedes the use of thiol based coupling chemistry. Antibody Fab fragments with an unnatural p-acetylphenylalanine residue could be used to create immunoconjugates and multimers to improve upon existing mAb therapeutics. In a proof-of-concept study, p-acetylphenylalanine was genetically encoded into the Fab of Herceptin trastuzumab at different locations and then coupled with aminooxy-biotin to form Fab multimers. In a human breast cancer cell line, the Fab multimers varied in their ability to inhibit the phosphorylation of HER2 (ERBB2, neu). Next steps could include using p-acetylphenylalanine residues to form conjugates between different biologics.