The synthesis of polymers made of amino acids has various potential applications such as the preparation of biomimetic biomaterials derived from natural proteins. One well-known method for producing peptide polymers is the polymerization of N-carboxy α-amino acid anhydrides, which was introduced by Leuchs at the beginning of the 20th century. The tendency of N-carboxy α-amino acid anhydrides to oligomerize was noticed by Leuchs himself, but the potential of N-carboxy α-amino acid anhydrides for giving access to large peptide polymers was established in 1947 with the work of Woodward and Schramm.
Showing all 16 resultsSorted by latest
-

Polycystine-poly(His,Arg)1:5 (CHR)
$ 150.0 Add to cart -
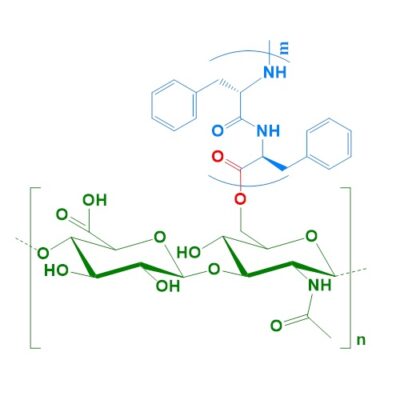
Phe-HA(ester)
$ 800.0 Add to cart -

γ-poly (glutamic acid)
$ 200.0 Add to cart -

Azelaic Acid-ε-PL
$ 480.0 Add to cart -

N-lauroylglycine-ε-PL
$ 360.0 Add to cart -

γ-PGA-Phe-OMe
$ 320.0 Add to cart -

γ-PGA-Trp-OMe
$ 360.0 Add to cart -

Poly-L-proline
$ 720.0 Add to cart -

Poly-L-Ornithine
$ 480.0 Add to cart -

Poly-L-arginine
$ 300.0 Add to cart -

Oligoarginine(R6,R7,R8,R9,R10)
$ 600.0 Add to cart -

Poly-D-lysine (PDL)
$ 600.0 Add to cart -

γ-poly (glutamic acid)
$ 210.0 Add to cart -

Poly-L-lysine (PLL)
$ 270.0 Add to cart -

ε-Poly-L-lysine (ε-PL)
$ 240.0 Add to cart -

Polyglutamine Q15
$ 660.0 Add to cart